"How to Anodize Steel" may been seen at https://www.youtube.com/watch?v=7g-azzYnMYo
This video describes the methods developed by Prof. Burleigh and his students for anodizing steel in hot caustic electrolytes. This process is the subject of two publications. The anodized layer is a nano-porous magnetite film that is adherent to the surface. The reflection of light from the top and bottom surfaces of the oxide film create a dichromic color that changes with viewing angle.
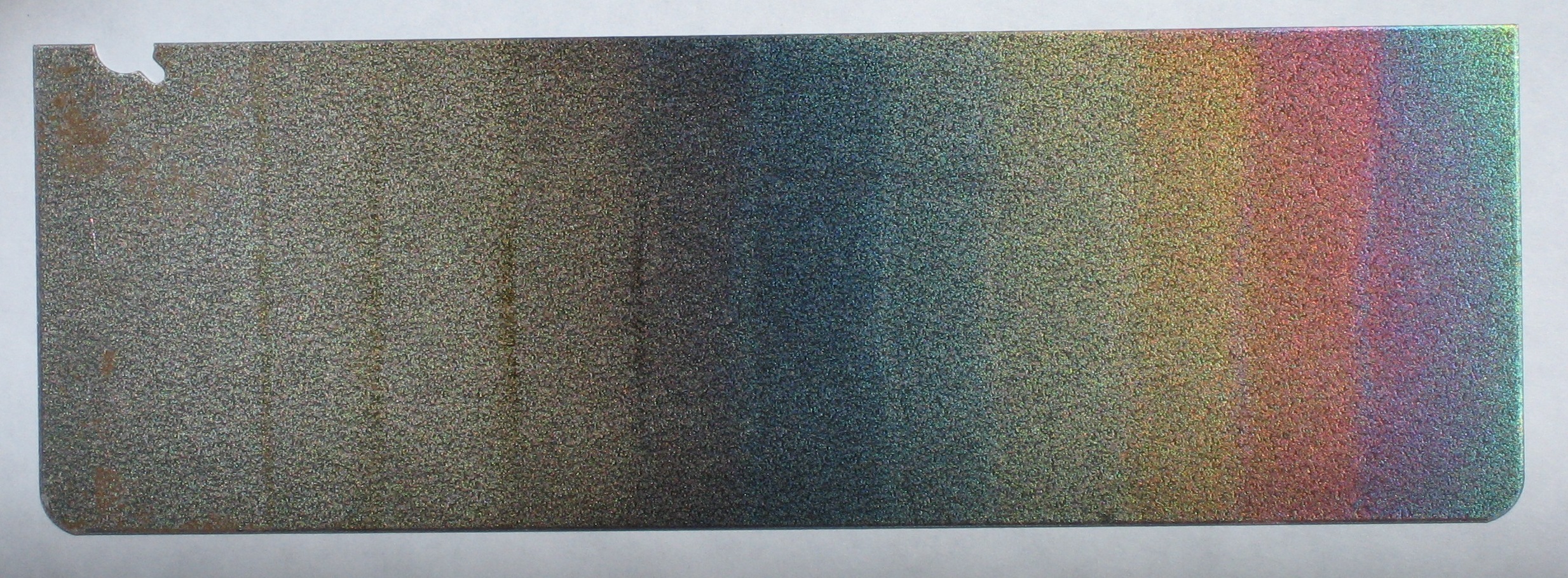
The dichroic oxide film has been grown on 1010 steel in 1 minute increments in 50% KOH solution at 70 C and +2.0 V vs. the steel counter electrode. (Fig 4 in Burleigh et al [2]).

A Damascus steel knife blade has been etched and then steelanodized. The different compositions of steel and the different heat treatments reflect different colors.

A steel rose was anodized by Brooks Robinson while a senior at RL Turner High School, Carrollton, TX, in 2023. The steel rose was cold-rolled mild steel, TIG welded together, bent with pliers, and polished before anodizing in 12.5 M NaOH at +2.5 volts.
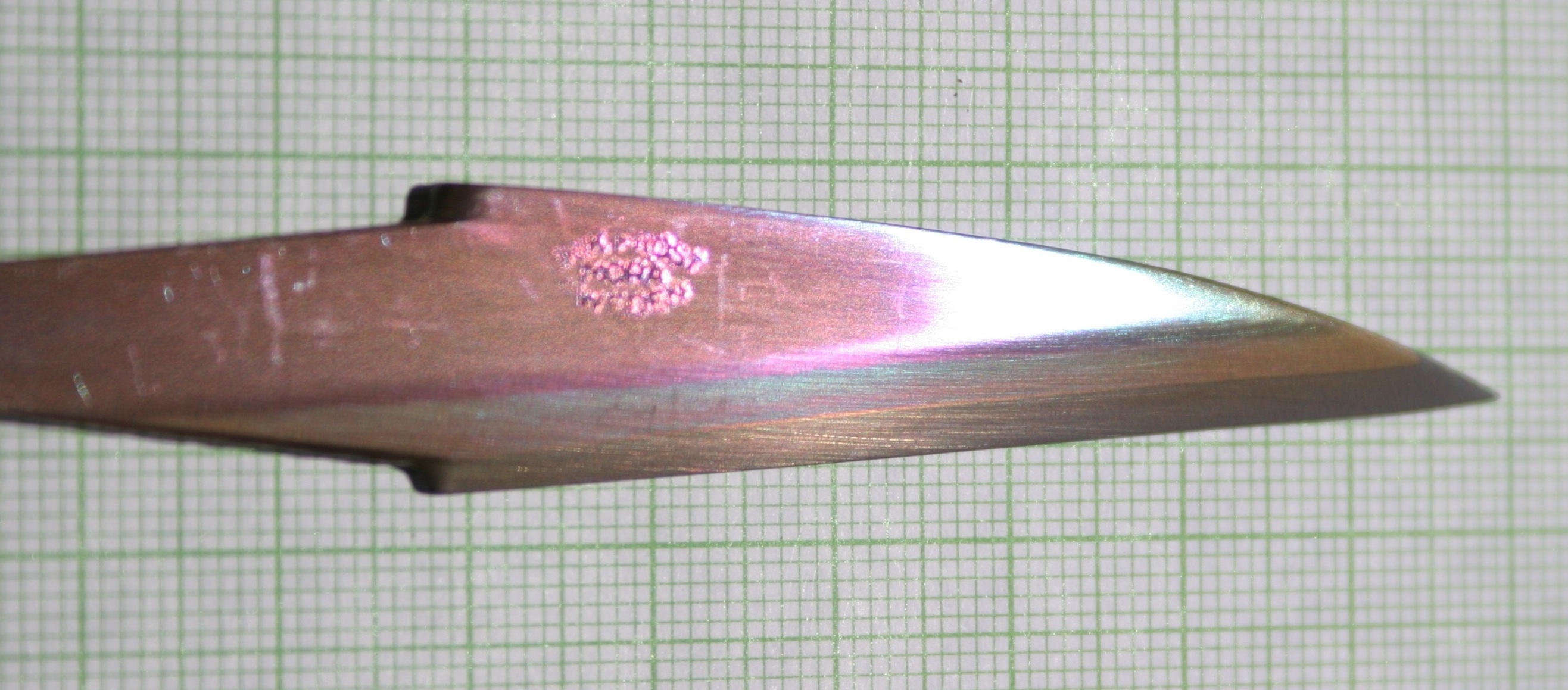
A dichroic knife blade made from layered carbon steel has been steelanodized. The steel layers have different compositions, which grow different oxide thicknesses, and therefore reflect different colors, giving the steel the rainbow appearance.
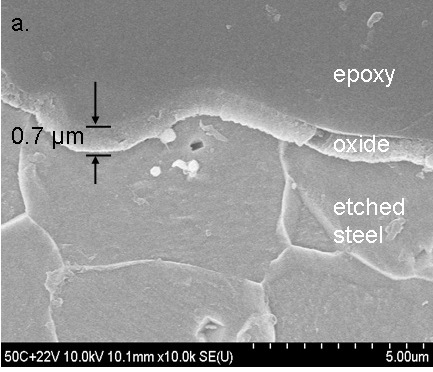
SEM cross-section of the anodic film grown on steel at 50 C and +2.2 V. (Fig 3a in Burleigh et al [3]).
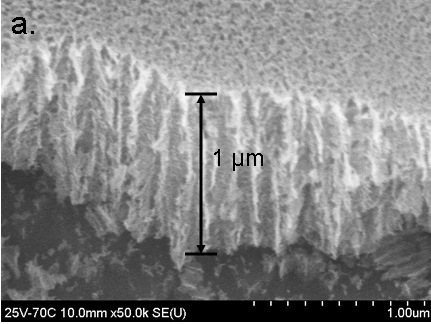
FESEM of the fractured oxide (70 C and +2.5 V) showing the nano-porous hollow channels. (Fig 8a in Burleigh et al [3]).
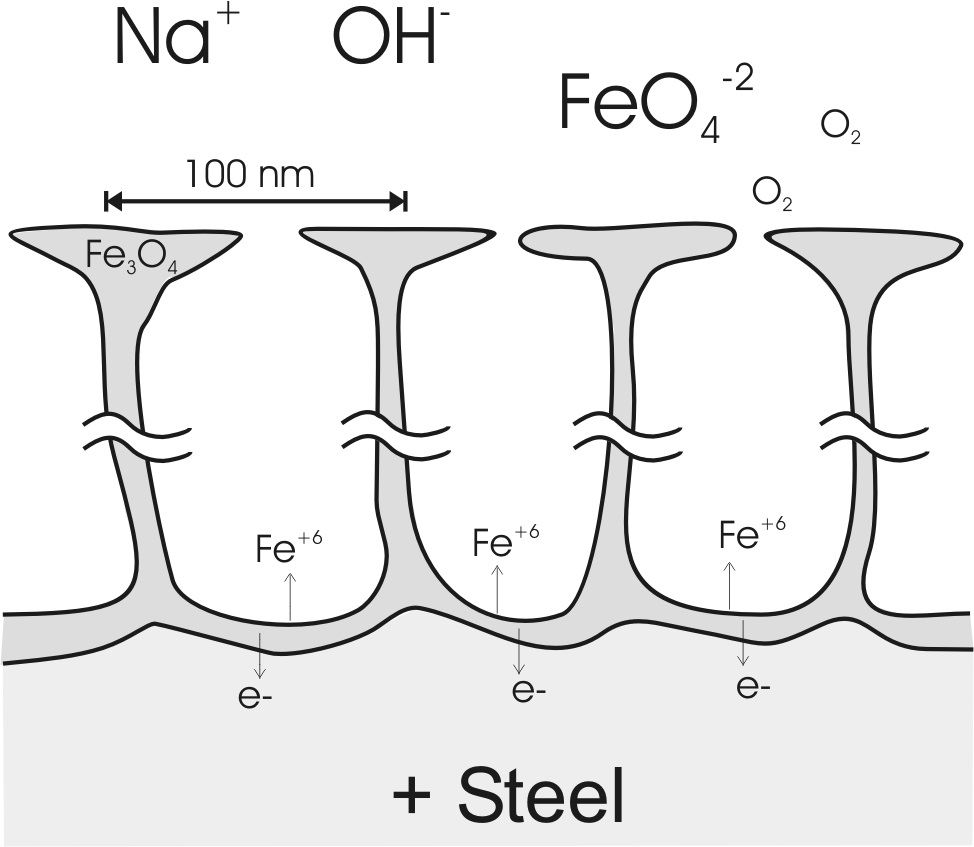
A model of the nano-porous magnetite (Fe3O4) channels which compose the anodic oxide. (Fig 10 in Burleigh et al [3]).
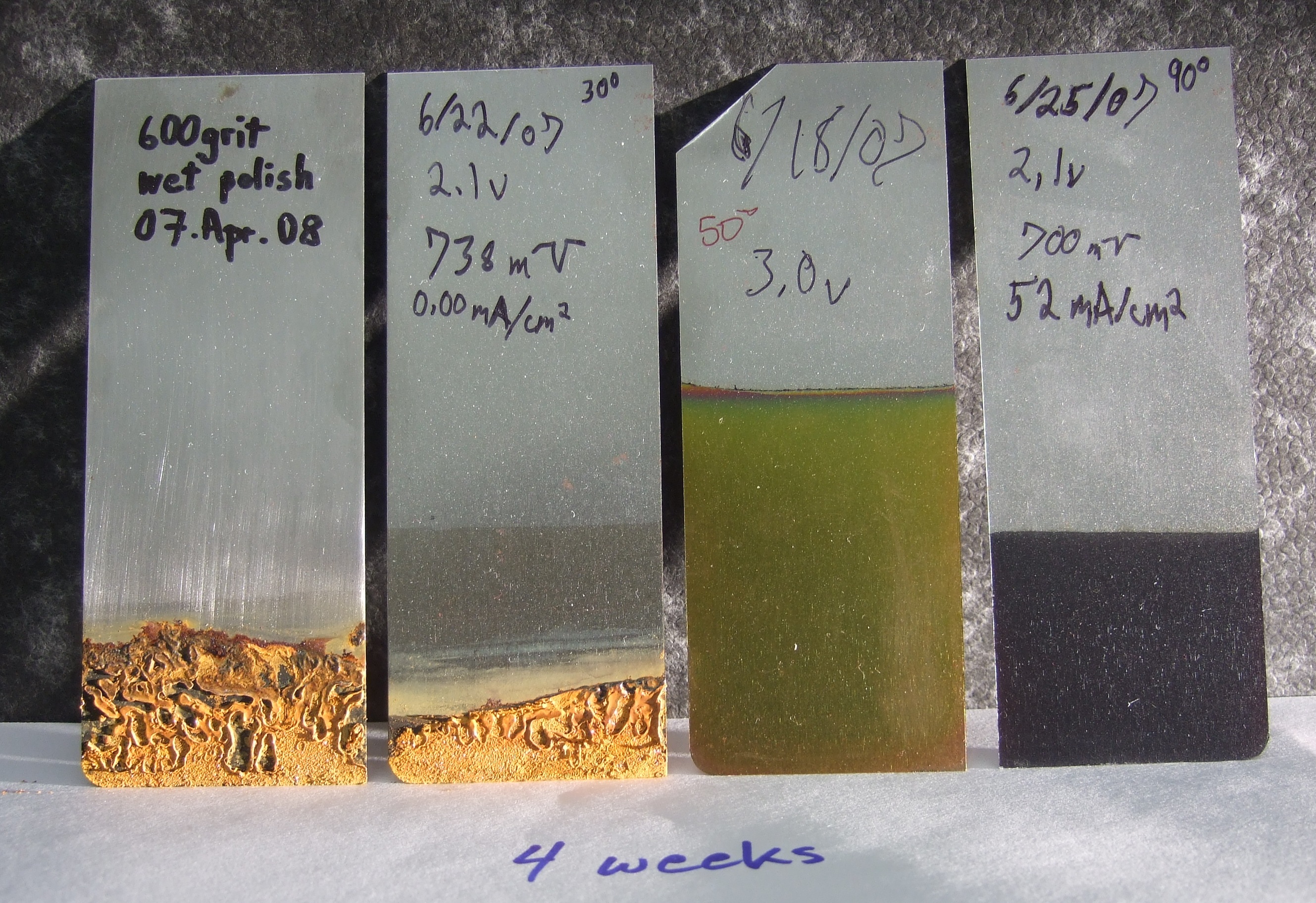
The steelanodizing provides corrosion protection against pure water. The four steel panels are shown after four weeks of partial immersion in 100 mL 18 MegaOhm-cm water after the water had evaporated. The far left panel was wet polished to 600 grit SiC prior to testing. The middle left panel was partially steelanodized. The two right panels were fully steelanodized at 50 C and +3.0 V, and at 70 C and +2.1 V respectively. (related to Fig 17 in Burleigh et al [3]).
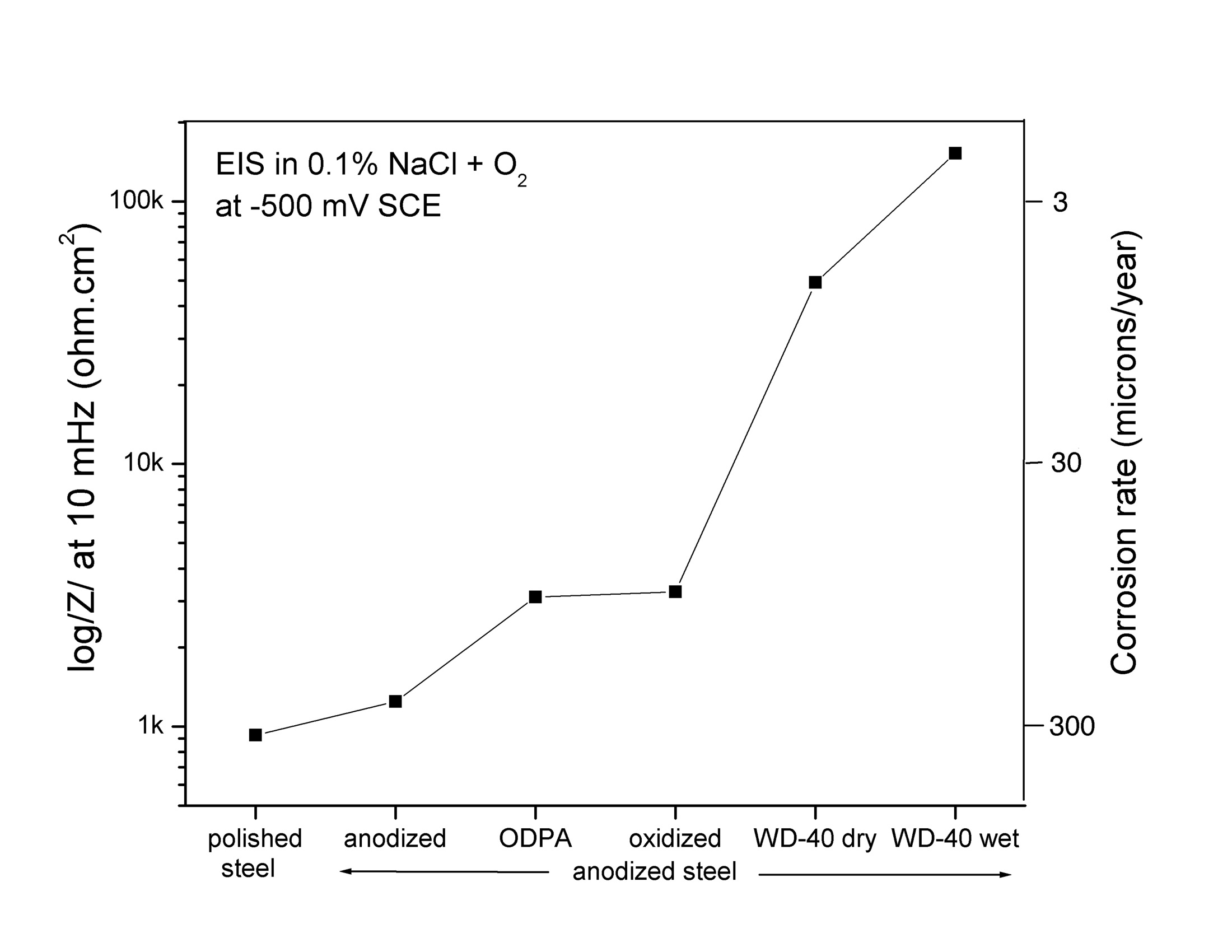
The steelanodizing provides corrosion protection against aerated saltwater if the pores are sealed with WD-40. The graph shows the reduction in the corrosion rate of steel with the different surface treatments. (Fig 18 in Burleigh et al [3]).
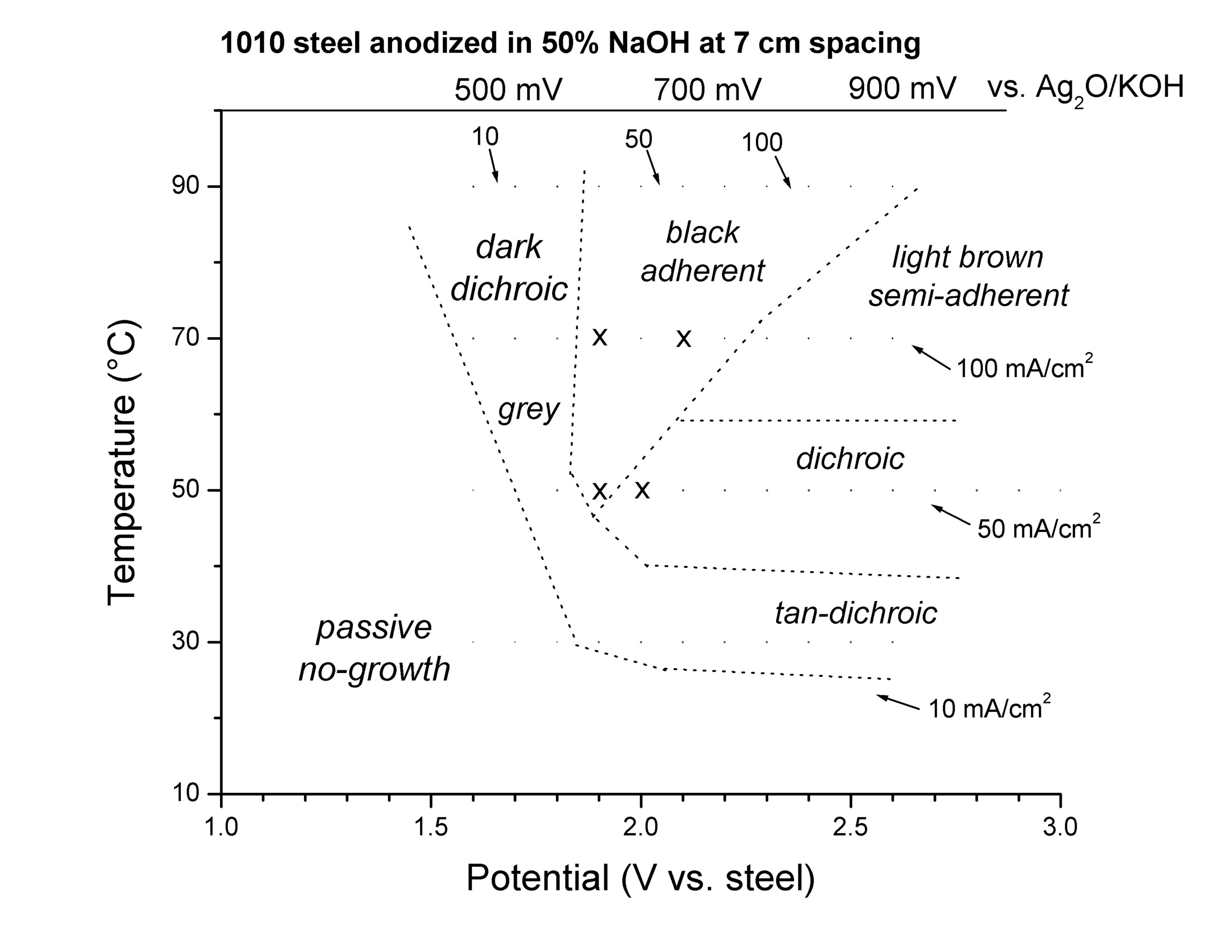
The steelanodizing can provide a large variety of different oxide films, depending on the temperature and the applied voltage. (Fig 1 in Burleigh et al [3]).